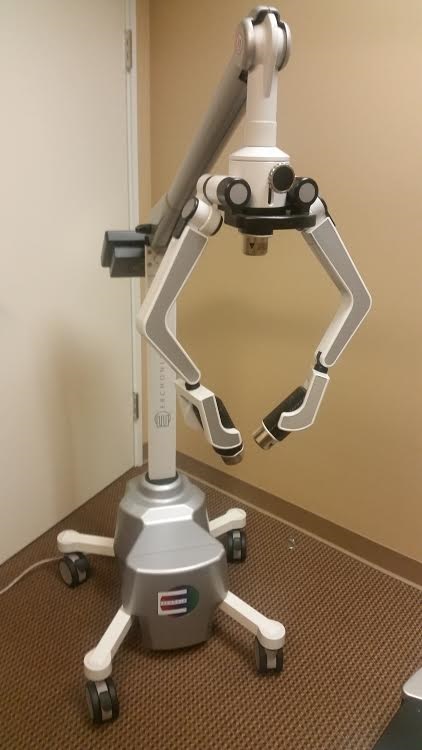
ERCHONIA FX 635 Laser Therapy
FX 635 – FDA Clearance to Treat Low Back Pain & Unattended Treatments
The FX 635 use low-level laser technology and patented laser diode arms to precisely target pain centers. It reduces inflammation while promoting bio-stimulation at a cellular level in the musculoskeletal point of the pain’s origination. Unlike other lasers that are unreliable and produce unwanted heat, the FX Series is fully compliant with ISO 13485 Medical Device Quality and IEC 60825-1 Laser Safety.
About FX 635 Laser
The FX 635 Laser is ERCHONIA flagship device for pain management. It is the only LLLT machine to be FDA Market Cleared for chronic heel pain arising from plantar fasciitis, and chronic low back pain of musculoskeletal origin.
The FX 635’s key features include: The quickest and most efficacious results as per clinical studies; completely automated so you can save on staff costs and increase profits; the best marketability of any pain relief laser in the world due to its US FDA-clearances.
How FX 635 Laser Works
FX 635 delivers parcels of light energy into the cell which the cell can use, and the parcels are delivered in the most effective and efficient way. This added energy fuels key processes, stimulating healing, relieving pain, reducing inflammation, and restoring function. For a more detailed explanation, please see the ‘What Is the Technical Explanation for How ERCHONIA Lasers Work?’ FAQ below.
Therapy Treatment
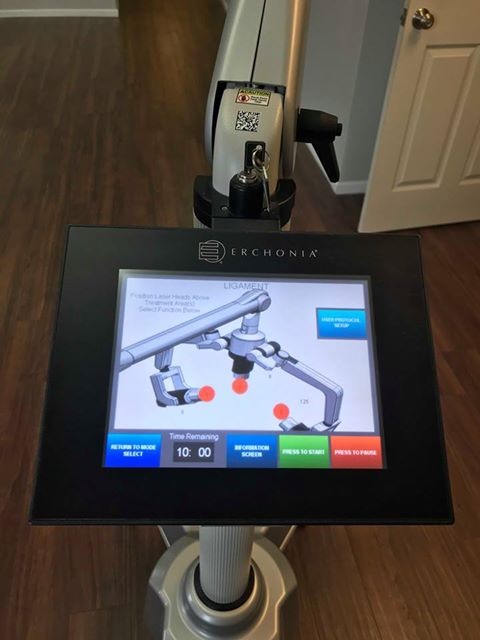
Users remain awake throughout the entire laser treatment and experience no burning, scarring or downtime, allowing them to resume the rest of their day as soon as the session is over.
ERCHONIA FX 635 Laser Therapy Specifications
Configuration: (3) Class 2 17.25mW Line Generating Diodes
Wavelength: 635nm
Modulation: Variable
Weight: 65lbs (29.48 kg)
Height: 70in (177.8 cm) (Average – Adjustable)
Two Independent Adjustable Arms For Desired Laser Concentration
4 Locking Anti-Static Casters
Display: Full Color Touch Screen Control
Power Source: 100-240VAC 50-60Hz
Chassis: Powder Coated Aircraft Aluminum
Housing & Covers: Non-Allergenic Material, Spray Finished
Accessories: 2-Keys, Power Cord, Laser Safety Glasses
Compliant to: ISO 13485 Medical Device Quality, IEC 60825-1 Laser Safety, IEC
60601-1-2 EMC, IEC 60601-1 Safety, CE Mark, CB Certified
FDA Laser Class 2 / FDA Device Class ll / EU Device Class IIa, Laser Class II










There are no reviews yet.